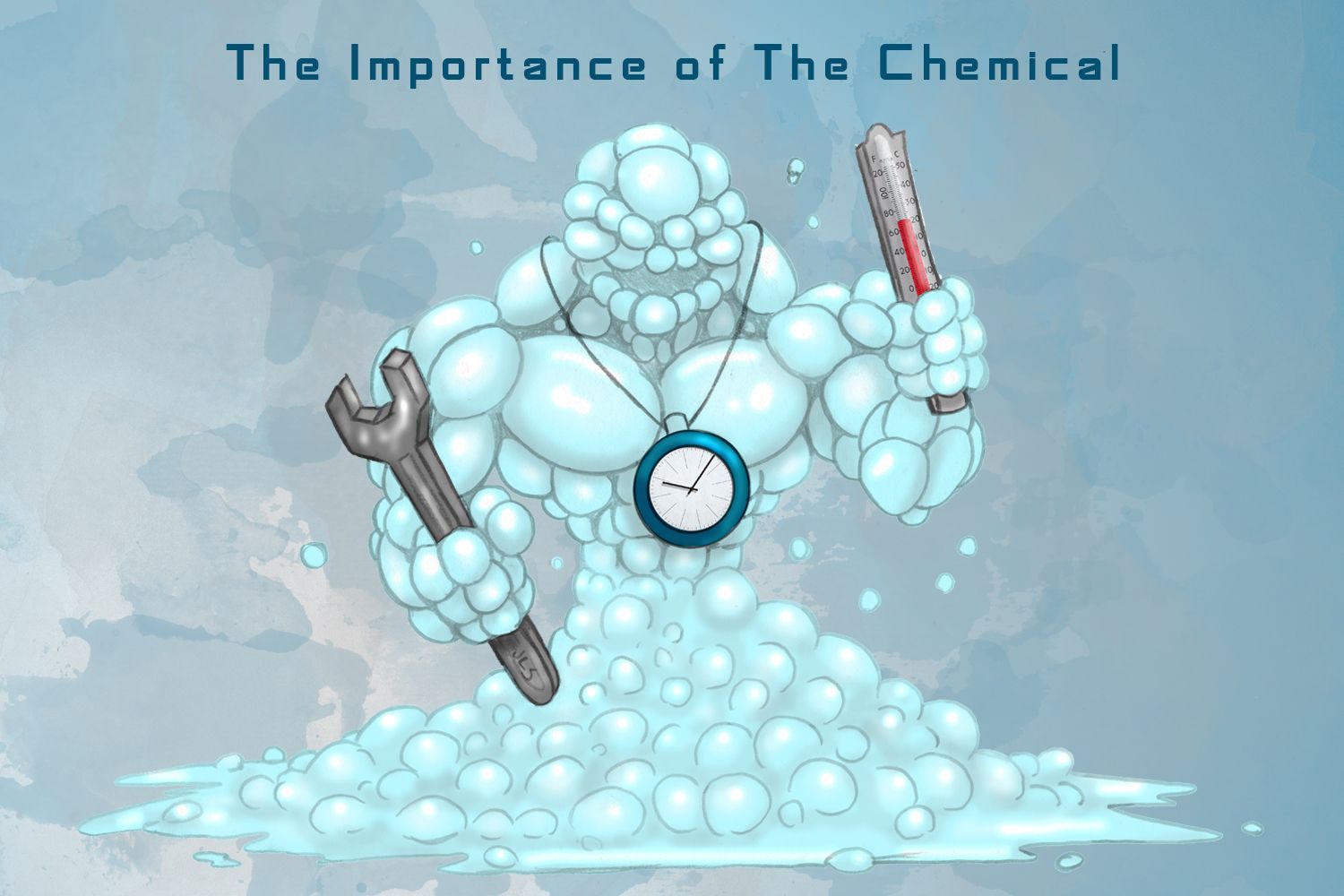
The Importance of The Chemical
There are vast amounts of chemicals on the market for the kitchen exhaust cleaning community. So many, in fact, that it can become difficult to choose which one is right for your company and your customers. Here at Omni Containment Systems , we know how important this choice is. Our goal today is to help describe the importance of the chemical in what is known as “The Sinner Circle” as well as discuss some of the science behind this essential chemical.
The Sinner Circle
The Sinner Circle is a cleaning concept where there are four distinct parts of the cleaning process: chemistry, mechanics, temperature, and time. The Sinner Circle was invented by Dr. Herbert Sinner in 1959 while working for the chemical company Henkel. His idea was much like Newton’s third law: every action has an equal and opposite counter action.
Each aspect of the circle is necessary for cleaning, but it can be modified for the process at hand. In the beginning of the process, all parts have equal share. However, if you increase one of the parts of the circle, then the other parts must decrease. Think of the circle as representing one hundred percent, with each part representing twenty-five percent. Because nothing can go over one hundred percent, if one of the parts increases its percentage of use, then the others must decrease equally.
The best example is washing a greasy dish. If you increase the heat of the water you are using (temperature), then you will not need to scrub it as much (mechanics). Also, you could add a stronger chemical (chemistry) and not need as much time for soaking. Finally, if you use less chemical and heat, then you must scrub harder and for a longer amount of time. The Sinner Circle simply states that there are many ways to clean but all methods require the four parts of the circle to some degree.
Chemistry
This is the type of chemical you are using and how it affects the grease you are trying to clean.
Mechanics
The physical factor in cleaning grease, such as water pressure, a scraper, or using Tegras -style brushes.
Temperature
Cold versus hot water, or using a chemical, such as sodium hydroxide, that is exothermic (gives off heat).
Time
Time in the sinner circle could mean different things, such as dwell time of the chemical you are using or total effort used to achieve a clean surface.
The Chemical Science
We are firm believers that the chemical is the most important part of the Sinner Circle. Being that it is the most important part, we need to understand the science behind these chemicals. Sometimes choosing the right chemical for your job can be tricky. The chemical supply world is vast with several similar compounds, giving you a choice between a few chemicals that will work for your project.
The two compounds that are often most often used by kitchen exhaust cleaners are sodium hydroxide (NaOH) and potassium hydroxide (KOH) ; both have fans and champions. Most kitchen exhaust cleaning contractors have settled on either sodium hydroxide or potassium hydroxide, or some combination of the two.
Sodium hydroxide and potassium hydroxide are almost interchangeable. They are the most chemically similar of the hydroxides, are both white, strong alkaline, and corrosive solid or powder. Sodium hydroxide is more commonly known as lye or caustic soda, and potassium hydroxide is known as potash.
Potassium hydroxide molecules are slightly smaller than sodium hydroxide molecules, meaning it cuts through oil faster than sodium hydroxide, breaking the oils hold on surfaces quicker. Potassium hydroxide is also more soluble, making it easier to rinse off a surface especially when using hot water. This makes potassium hydroxide a great choice when you need to remove hard, baked on oil or grease.
Potassium hydroxide and sodium hydroxide both have a strong exothermic process, meaning they dissolve/dissociate in water giving off ions to create heat. Although, sodium hydroxide has a slightly higher exothermic process which can make up for other, more positive, factors that potassium hydroxide possesses (like having smaller molecules). The extra exothermic edge of sodium hydroxide works well when cleaning thick, fatty, and soft grease and oil, especially if using colder water.
Due to production costs, potassium hydroxide is generally more expensive than sodium hydroxide. Both chemicals are produced through a process called electrolysis. Sodium hydroxide is produced using sodium chloride, otherwise known as plain table salt. Potassium hydroxide is made using potassium chloride, a more costly compound; a unit “ton” of potassium hydroxide is about three times more expensive than sodium hydroxide.
Potassium Hydroxide (Potash)
- More expensive
- More soluble than sodium hydroxide
- Molecularly smaller than sodium hydroxide
Sodium Hydroxide (Caustic Soda)
- Less expensive on average by a factor of 3
- Slightly more exothermic, making it give off more heat
- Molecularly bigger than potassium hydroxide
Conclusion
In summary, if you are needing to clean a metal surface that is coated with a hard, baked on oil or grease, you may prefer to use potassium hydroxide with a hot water rinse. Whereas a coating of thicker or softer oil or grease could be cleaned by using sodium hydroxide for less cost with lower water temperature required because of the better exothermic process.
Using a product that contains both chemicals is like getting the best of both. You typically can get a lower priced product with a better exothermic process than with potassium hydroxide alone, but you will have faster penetration and a better rinsing product than if it just had sodium hydroxide alone. This gives you a chemical that shocks grease off the surface while also dissolving it with exothermic heat at a middle range price. If you have any questions, ask our team ! Omni Containment Systems is always ready to help our customers.
Share This Blog Post!



The Role of TEINNOVA in Revolutionizing Duct Cleaning: A Collaboration with Omni Containment Systems
















